Fundamental Concepts
Certain fundamental concepts are given below to understand biological (living) systems –
We are able to perceive the material world around us through five senses. 1) Vision 2) Hearing 3) Smell 4) Taste 5) Touch. Technological advances have helped us tremendously to extend its applicability. For example, electron microscope assists us to visualize an object with utmost details.
Consideration can be given to following components while studying anything.
1) Structure, Form, Function –
Structure means the matter from which anything is made. Everything material has some quality which can be termed as its function. Form denotes appearance or the arrangement of something.
Structure, form and function are interrelated. Take an example of a chalk we use to write on the blackboard.
It is made up of calcium carbonate – it’s structural matter. It is used for writing – it’s function. Generally, it is 2-3 inches lengthwise, circular in shape and white in colour. This is its form.
Now you make the chalk red coloured. Structural matter has changed due to addition of some chemical which changes the colour to red. Form or the appearance has changed. It is possible that we use red coloured chalk to write something specific. It means functional aspects have also changed.
2) Quality, Quantity –
Quality means a distinct feature, like particular odour, colour , taste etc. As you will notice, it is subjective. Subjective means it may differ from person to person. A may say the odour is very strong while B may say it is pleasant!
Quantity denotes specific amount which can be measured and expressed in number. Therefore, it is objective. It means there can not be two opinions on it.
3) Time, Space and Change –
It is difficult to define time. It is a moment with reference to which one talks about. Space means three-dimensional area within which any physical object(s) exists. Change is the fundamental aspect of the world around. It denotes difference and it occurs constantly in relation to structure, form, function, quality and quantity. It is called as temporal (time) and spatial (space) dimensions while understanding the material world. Change is occurring at every moment at all levels from atom to cell to living and nonliving world. Dynamism is the word which denotes it in nutshell.
Let us try to understand the biological systems on this background as human system belongs to biological world.
System –
The word system has a technical significance. A system can be defined as an assembly of units joined together in a particular manner to perform a specific function. It needs energy (input) to perform the function. The function is assessed (measured when one talks about an engineering system), and if error is noticed one can look into input or interrelated units in the system to rectify it.
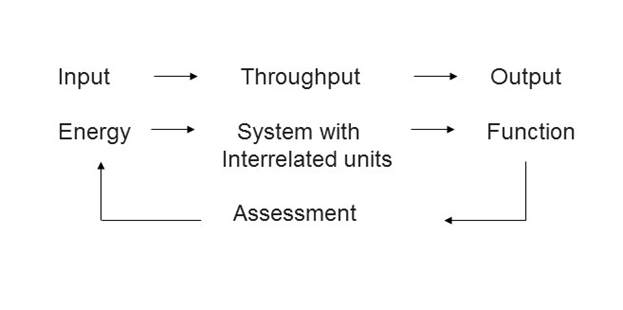
Biological system –
Biology means scientific study of living organisms. From evolutionary point of view, over billions of years back, earth arrived on the scene of this universe. Later number of chemical elements, compounds and subsequently life arose in the form of simple microorganisms. The living world differs from nonliving in that it could extract energy from
surroundings, grows and has capacity to form new versions. An overview of a few concepts in chemistry is necessary at this juncture.
An atom is the smallest possible particle of an element that retains the characteristics of the elements. Atom consists of three basic sub-atomic particles. In the centre there is nucleus consisting of positively charged protons and neutrons which have no charge. Surrounding the nucleus are negatively charged electrons moving in orbits. The number of electrons is equal to number of protons so that the overall charge is zero. The word
charge denotes electrical energy.
An element is a substance which can not be reduced further by ordinary processes and is made up of same kind of atoms. There are 92 naturally occurring element
Molecules can be defined in simple terms as a group of atoms existing together as a unit with characteristic properties. In molecule, two or more atoms of the same element are bound together through chemical bonding. Bond refers to an attractive force that keeps a group of atoms together. Attractive forces in bonding are positive charges on the nucleus
and the negative charges on the shared pairs of electrons.
Compounds are molecules where two or more atoms of different elements are combined.
For example –
Na (2. 8. 1) + Cl (2. 8. 7) —- Na+ (2. 8) + Cl –(2. 8. 8)
Sodium has one electron in the outermost shell while chlorine has seven. In the formation of sodium chloride (ordinary salt in our food) molecule, sodium loses one electron while chlorine gains one. The two atoms bond together by ionic bond. The resulting charged species are called ions held together by electrostatic attraction.
Ion means an atom or molecule which has a resultant electric charge due to loss or gain of valence electrons making it either positively charged (cation) or negatively charged (anion). For ex. in the above example of sodium chloride, Na is a cation while Cl is anion.
The word valency denotes capacity of an atom of a given element to combine with or replace atoms of hydrogen which has single electron in its shell. Depending on valency different type of bonding is possible. For ex.
Chlorine can form single covalent bond with another chlorine atom.

Oxygen with its six electrons in the outermost shell form double covalent bond.
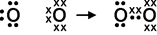
Biomolecule –
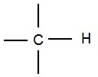
It is a molecule that is involved in the maintenance and metabolic processes of living organisms. Biomolecules are compounds of carbon with various functional groups attached to it. Carbon is tetravalent. It means it has a valence of four. One can imagine carbon as universal friend collaborating with other atoms or groups to form thousands of compounds. Carbon can form single bonds with hydrogen.
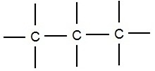
Carbon can form stable bonds with other carbon.
Most bio molecules are derivatives of hydrocarbons with hydrogen atom replaced by a variety of functional groups.
For eg. Alcohol with hydroxyl group
Amines with amino group
Aldehyde and ketone with carbonyl group
Carboxylic acid with carboxyl group
Carbohydrates –
Carbohydrates are most abundant biomolecules in the living systems. They are also called as Saccharides, a word originated from Greek meaning sugar. Simple carbohydrates such as glucose and fructose are sweet. Polysaccharides and oligosaccharides such as glycans are monosaccharaides units in long chain with branching. For e.g. Starch in plants, glycogen which stores energy in bacteria and animal cells, cellulose in plants providing rigidity to plant cells. There are number of other carbohydrates such as peptidoglycans, glycosaminoglycans etc.
In animals, oxidation of carbohydrates is the central energy providing process. Carbohydrates can act as information molecules (see further) in cell-cell interaction.
Lipids –
Lipids are diverse group of compounds their common property being insolubility in water. Fats and oils which are lipids are a major form for storage of energy. Few lipids act as signals, means trigger of a specific reaction in the system. For ex. Prostaglandins derived from arachidonate, participate in many cellular functions.
Proteins –
Derived from 20 amino acids proteins form the most important compounds in the living systems. Covalent bonds of amino acids give rise to peptides from which proteins are formed. Different combinations of amino acids with differing spacio temporal relationship give rise to thousands and thousands of different proteins. Some proteins contain other chemicals like lipoproteins with lipids, glycoproteins with sugar group, metalloproteins with metals. Proteins are very flexible. Protein synthesis, folding and refolding of proteins along with degradation of proteins which have not been properly folded occur simultaneously so as to maintain balance. In cells, the process involves hundreds of enzymes and specialized proteins. The functioning of many proteins depends on their capacity to bind reversibly to other molecules. Such molecules are termed as ligands.
Entropy, Informatics, Bioenergetics:
Entropy means molecules have an inherent tendency for randomness and disorder. However, in living systems macromolecules are formed and interact in a specific manner. It requires order and balance.
This is achieved by “Information” carrying molecules, mostly in the form of proteins. The term information refers to the degree of freedom that exists in a given situation to choose amongst symbols or the matter required for a specific work. What does it mean? Imagine you are at a junction where 5 roads meet. Your destination can be approached by 3 of them. You will choose one which is cost effective. Now you put yourself at some other
location in one of these roads. Your choice will differ. Similarly, in a cell, a specific function can be performed through different cascade of bio molecular interaction. The choice and orderly functioning are achieved by information carrying molecules. The amount of energy and the enzymes required for it are much larger than simple chemical reaction.
Interdependence of living systems and energy flow:
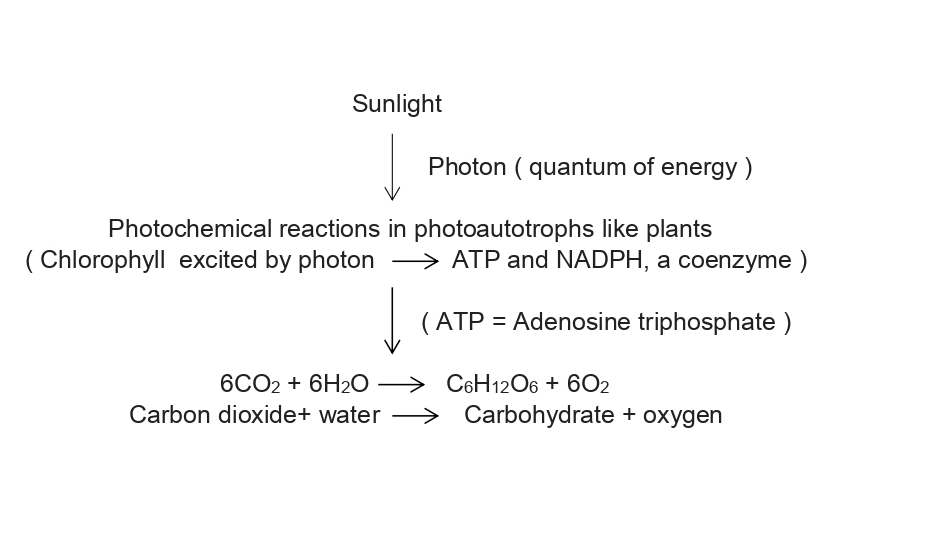
Thus, energy in the form of ATP is used to make carbohydrates and other compounds from carbon di oxide and water releasing oxygen in the atmosphere. It is called as phosphorylation wherein ATP provides energy and gets converted to ADP (Adenosine diphosphate)
As against it in non-photosynthetic organisms i.e chemotrophs, energy is obtained by oxidizing carbohydrates produced by plants with release of carbon di oxide in the atmosphere along with production of energy and water.
C6H12O6 + 6O2 ——- 6CO2 + 6H2O + Energy
It is called as oxidative phosphorylation which occurs in mitochondria ( i.e. enzymatic
phosphorylation of ADP to ATP ) coupled to electron transfer from a substrate to
molecular oxygen. Substrate means a molecular species upon which an enzyme acts.
Oxidation Reduction Reaction:
It can be simply defined as addition of oxygen or removal of Hydrogen, both are complementary. The substance that provides oxygen or removes Hydrogen is the oxidizing agent. The substance that provides Hydrogen or removes oxygen is the reducing agent.
As noted earlier in plants Co2 is reduced while in animals, oxidation of glucose occurs with release of Co2. Thus, in the living world coupling of oxidation reduction reactions provide energy, the primary source being sunlight.
